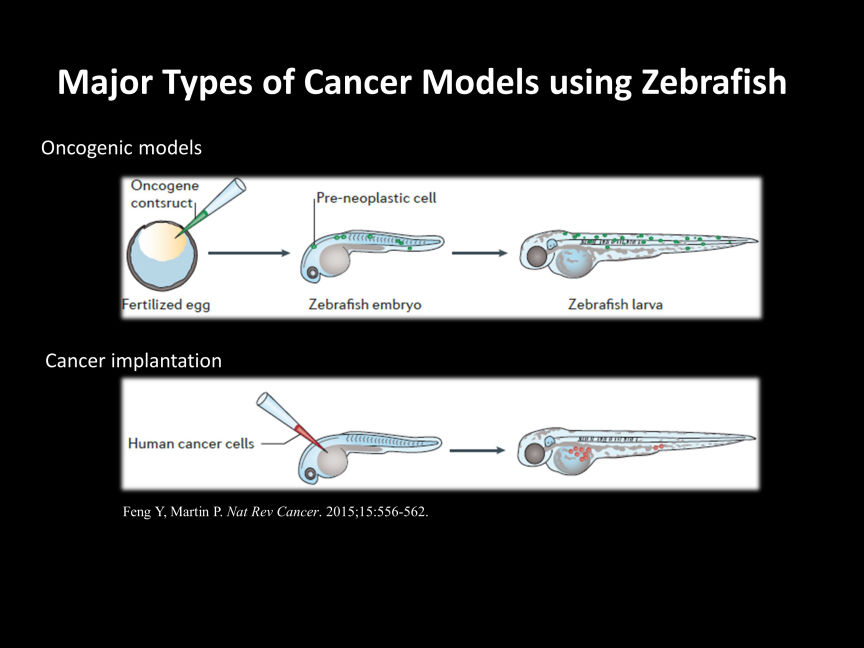
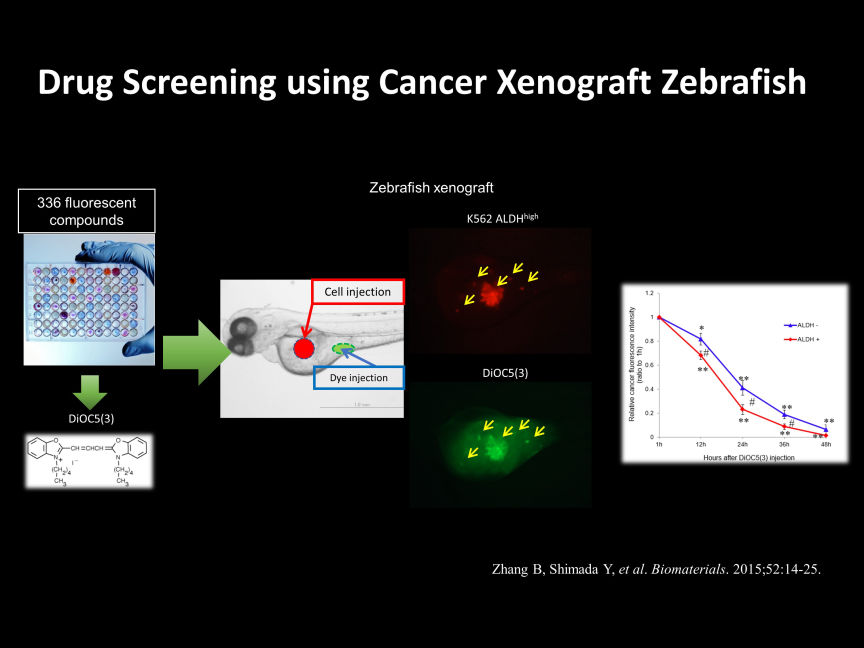
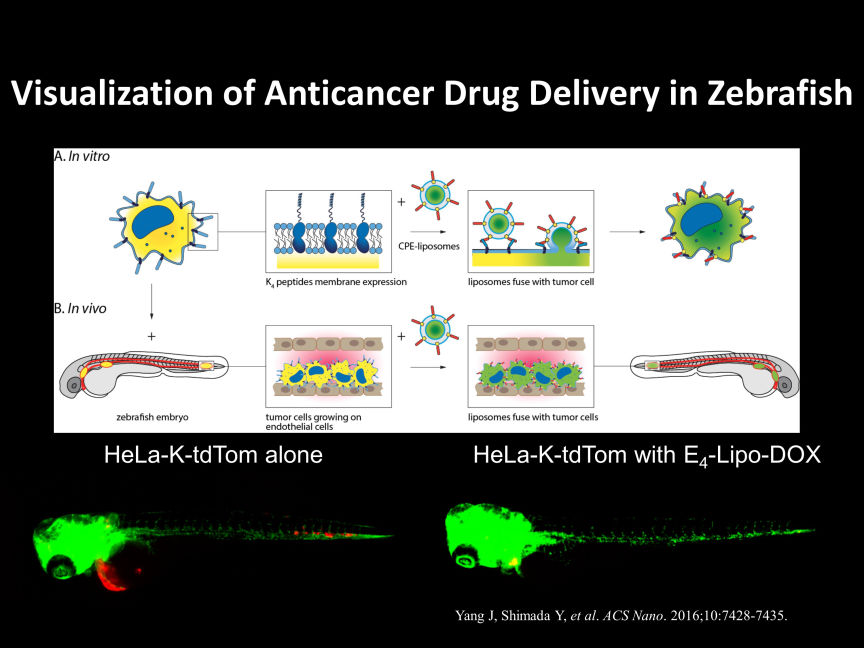
There are various methods for cancer research using zebrafish, but mainly two approaches are considered major.
1. Oncogenic models
By overexpressing oncogenic genes from humans or zebrafish in target organs, or by deleting tumor suppressor genes, zebrafish can be induced to develop cancer. Models that introduce the well-known BRAF (V600E) mutation gene associated with malignant melanoma into the skin of zebrafish, or liver cancer models, are commonly used.
2. Cancer implantation
This is a model where cancer cells derived from humans or other animals are transplanted into zebrafish. Success rates vary greatly depending on the transplantation site (yolk sac, duct of Cuvier, perivitelline space, intraperitoneal), stage of zebrafish (larvae, young, adult), and type of cancer cells used for transplantation, but there are numerous reports on this approach.